Urea, with the CO formula (NH2)2is a chemical compound which is widely used in a range of sectors, including manufacturing, agriculture and various industries. Conventionally, this compound is produced via a two -step process which implies the synthesis of ammonia from nitrogen (N₂) and its subsequent reaction with carbon dioxide (CO₂).
This reaction occurs at high temperatures and high pressure, leading to the formation of a compound called ammonium carbamate. This compound is then broken down to lower pressures, which ultimately produces urea and water.
The traditional urea production processes are very important in energy, which means that to produce desired quantities of urea, they consume a lot of electrical energy. In recent years, some engineers have thus tried to design more energy efficient strategies to synthesize urea.
A possible approach could be to directly synthesize Co₂ and N₂ urea using electrolysis, devices that use electricity to facilitate desired chemical reactions. Until now, the use of these devices to synthesize urea has proven to be difficult, as secondary reactions not sought in the devices often produce other compounds instead.
Researchers from the Sun Yat-Sen University in China have recently introduced a new strategy to synthesize pure urea from pre-treated combustion gas, a waste gas emitted by industrial processes, in a proton-limited environment reached using an electrolyzer which incorporates an electrolyte in the solid state. Their article, published in Nanotechnology of naturecould open new valuable opportunities for the production of large -scale energy -efficient urea.
“The electrosynthesis of pure urea via the co-reduction of CO2 and n2 It remains difficult, “wrote Yan-Chen Liu, Jia-Run Huang and their colleagues in their article. We show that an environment limited to a proton established in an electrolyte equipped with an electrolyte with a porous solid state, devoid of an aqueous electrolyte, can remove the evolution reaction of hydrogen and excessive hydrogenation of N.2 to ammonia.
-“It can rather be conducive to coupling C – n of * co2 With * NHNH (the intermediary of the semi-hydrogenation of n2), thus facilitating the production of urea. “”
The new strategy to synthesize the urea introduced by this research team is mainly based on the creation of a limited protons environment, a condition in which hydrogen ions (i.e. protons) are rare. This condition was successfully carried out using an electrolyzer which contains an electrolyte in a porous solid state.
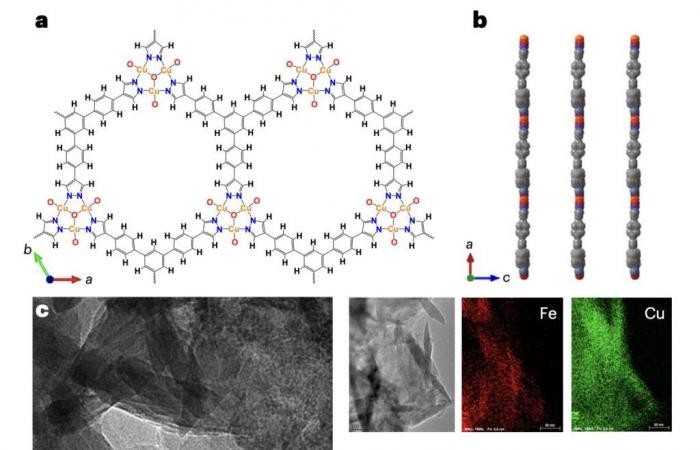
“Using nanofeuilles of a metallic frame-Azolate bidimensional ultra-dimensional with cyclical heterotrial clusters as a catalyst, the faradaic efficiency of urea production from pre-treated combustion gas (which contains mainly 85% N.2 and 15% co2) at a height of 65.5%, and no ammonia product and other liquid products have been generated, “wrote Liu, Huang and their colleagues.
“At low cell voltage of 2.0 V, the current can reach 100 mA and the production rate of the urea is high that 5.07 gchat−1 H−1ou 84.4 mmol gchat−1H−1. In particular, it can continuously produce an aqueous solution of pure urea of 6.2% by weight for at least 30 h, and approximately 1.24 g a solid pure urea was obtained. “”
In the initial experiences of the team, their strategy has enabled continuous production of high purity without perproducts of ammonia, while consuming less energy than conventional summary approaches to urea. In the future, it could be tested more and implemented on a large scale, potentially allowing the greener and profitable production of large -scale urea.
“The use of pre -treated combustion gas as a direct raw material considerably reduces the costs of the inputs, and the high reaction rate and selectivity contribute to a reduction in the system scale and operational costs,” the researchers wrote.